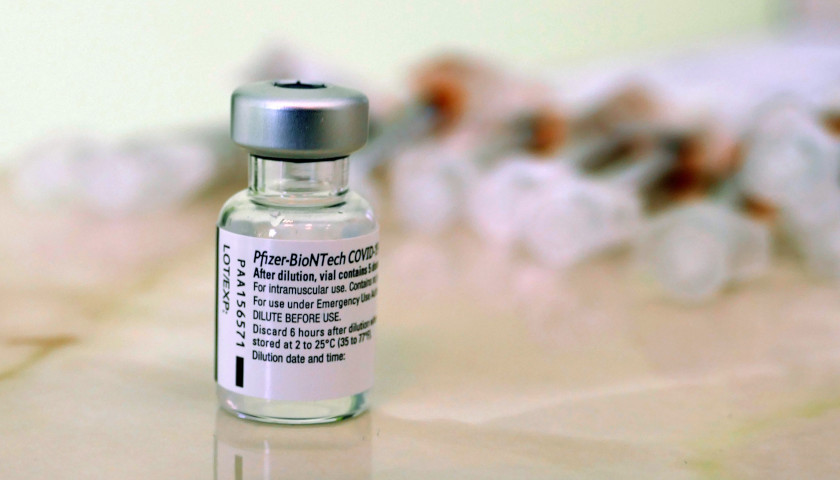
The Pfizer BioNTech COVID-19 vaccine authorized under the Emergency Use Authorization (EUA) has a different warning about certain heart health risks than the fully FDA-approved Comirnaty version of the vaccine.
The Pfizer BioNTech fact sheet has been updated throughout the year. A June 10, 2021 CDC presentation first noted myocarditis and pericarditis reports following both Pfizer and Moderna vaccinations.
On June 25, the fact sheet was revised with a section on the complications:
Myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the lining outside the heart) have occurred in some people who have received the Pfizer-BioNTech COVID-19 Vaccine. In most of these people, symptoms began within a few days following receipt of the second dose of the Pfizer-BioNTech COVID-19 Vaccine. The chance of having this occur is very low. You should seek medical attention right away if you have any of the following symptoms after receiving the Pfizer-BioNTech COVID-19 Vaccine:
• Chest pain
• Shortness of breath
• Feelings of having a fast-beating, fluttering, or pounding heart.
Meanwhile, the Comirnaty label vaccine includes a package insert that states:
Postmarketing data demonstrate increased risks of myocarditis and pericarditis, particularly within 7 days following the second dose. The observed risk is higher among males under 40 years of age than among females and older males. The observed risk is highest in males 12 through 17 years of age. Although some cases required intensive care support, available data from short-term follow-up suggest that most individuals have had resolution of symptoms with conservative management. Information is not yet available about potential long-term sequelae. The CDC has published considerations related to myocarditis and pericarditis after vaccination, including for vaccination of individuals with a history of myocarditis or pericarditis (https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html).
Comirnaty’s myocarditis risk is significantly more detailed than the risk of the same adverse side effect in the EUA authorized version of the vaccine. The EUA version of the vaccine was also issued well before the risk of myocarditis was known.
But Pfizer confirmed to The Star News Network last week that it is not currently shipping Comirnaty in the United States. The vaccine doses that are being administered are only the EUA-authorized version of the vaccine.
Pfizer has said it will continue to distribute that vaccine until stock runs out.
An FDA official told The Virginia Star said that “the FDA has to ensure that EUA vaccine recipients are informed of the EUA, the extent and benefits of the vaccine, that the vaccine is optional, and of alternatives to the vaccine. Normally that data is communicated through a fact sheet for EUA vaccines.”
A package insert is used with fully approved vaccines like Comirnaty.
The FDA has said the vaccines are “interchangeable” with certain differences that don’t impact safety or effectiveness, but that the two products are legally distinct. Although Pfizer also says the vaccines are “interchangeable” and that they have the same formulations, at least one commentator has challenged the pharmaceutical maker’s assertion.
Reuters reported that New Zealand officials linked the death of a man to myocarditis, within two weeks after his first dose. The man had not sought medical treatment; he was New Zealand’s second fatality from myocarditis linked to the vaccine. It remains unclear which version of Pfizer’s vaccine is being distributed in New Zealand.
“Both RNA vaccines – (i.e. Pfizer and Moderna) carry warnings about myocarditis and pericarditis risks,” University of Virginia Professor of Law Margaret Riley told The Star. She added, “Incidentally, the risks of myocarditis and pericarditis are far less with any of the vaccines than with contracting COVID itself.”
Professor Riley continued, “You click on the warnings when you sign up for the vaccines (on line obviously) – I don’t know how they give you the warnings if you sign up in person, but I’d assume that they have fact sheets. In my own experience, if you are over 17, you are informed that the vaccine is now fully approved, but you wouldn’t know the lot # (and therefore whether the actual vaccine was produced before or after the full approval) until you show up for your vaccine.”
Riley concluded, “I don’t think any of this raises legal or ethical issues since the actual vaccines and warnings are identical. CDC provides details about adverse events that have been reported on its website.”
But the difference between the EUA approved vaccine and Comrinaty does raise legal questions.
Federal government vaccine mandates, including the vaccine mandate for all military personnel, were predicated on the assertion that Pfizer’s fully-approved Comirnaty was available to the public. It is not.
Several lawsuits over this very issue the state are currently being adjudicated in the state of Ohio, where a law prevents public universities from mandating vaccines that are not fully approved by the FDA.
Ohio’s public schools have all mandated vaccination for their students, despite the fact that students can only procure EUA-authorized versions of the vaccine.
– – –
Eric Burk is a reporter at The Virginia Star and The Star News Network. Email tips to [email protected]. Pete D’Abrosca contributed to this report.