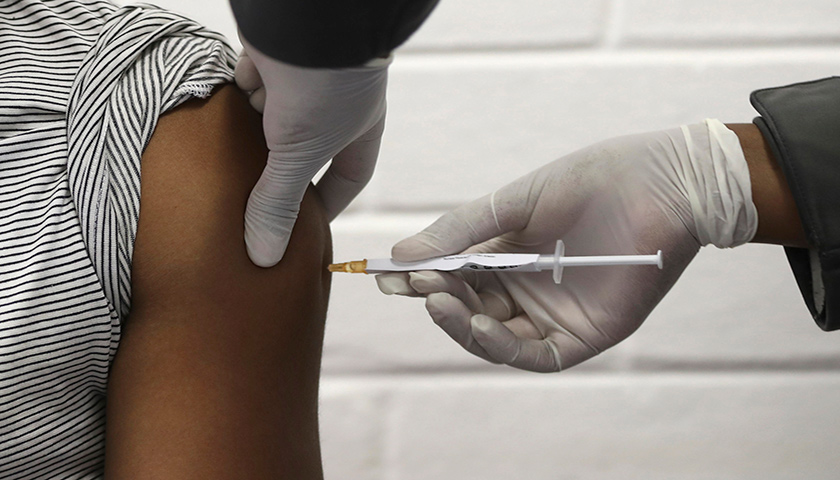
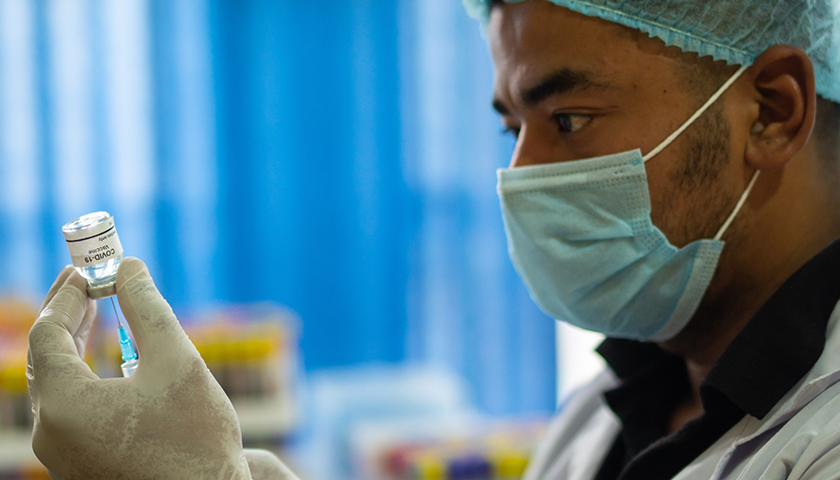
Governor Ralph Northam announced Monday that Virginia has reached a key vaccination milestone: 70 percent of adult Virginians have received at least one dose of a COVID-19 vaccine.
“Virginia has reached a significant milestone in the fight against COVID-19,” Northam said in his announcement. “Thanks to the millions of Virginians who have rolled up their sleeves to get vaccinated, the virus is in retreat, our economy is growing, and we are closer to putting this pandemic behind us.”
Read More