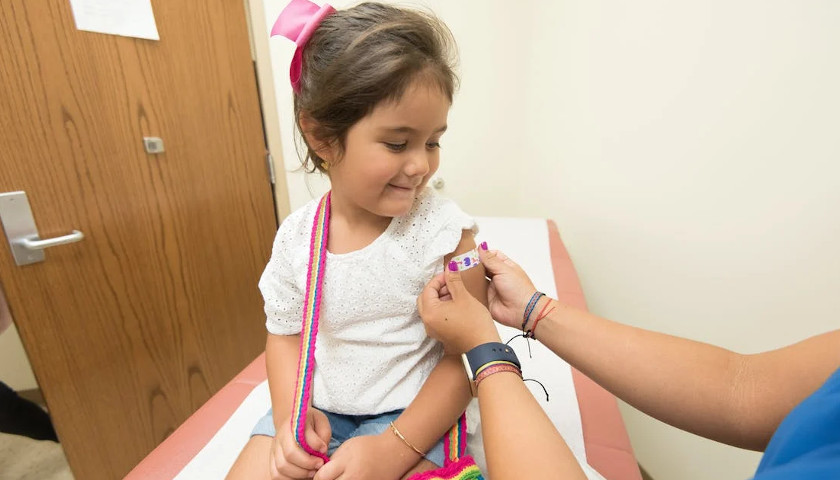
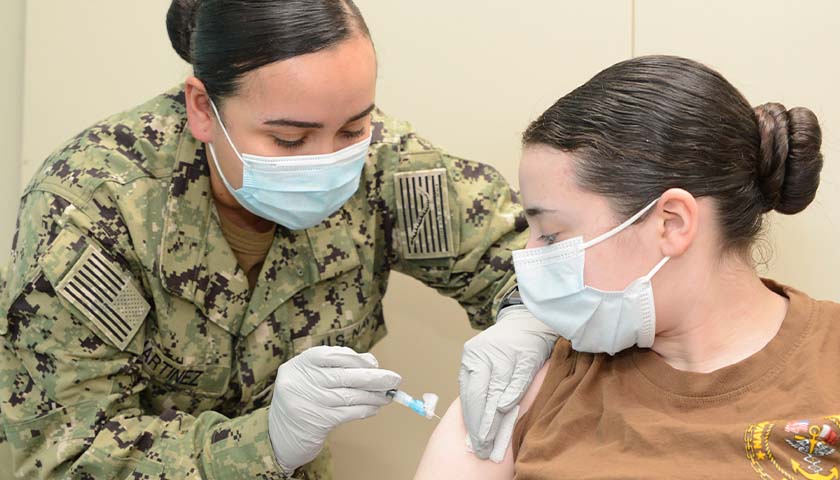
On August 24, Secretary of Defense Lloyd Austin issued a memo to senior Pentagon leadership announcing that he was implementing a mandatory COVID-19 vaccination policy for all military service members. The day before, the FDA had issued full authorization to Pfizer for their Comirnaty COVID-19 vaccine product (the nomenclature of which is meant to be a mashup of the words “COVID”, “mRNA”, and “community”) . At first glance it would seem that the mandatory vaccination policy, while scientifically unsound and strategically foolish, was at least a policy being implemented according to both the letter of the directive and in accordance with the law. But a further examination of the facts and the manner in which this order is being implemented makes clear that the military’s implementation of this order is illegal and highly unethical.
In the memo, Secretary Austin issued a directive and a promise, that “Mandatory vaccination against COVID-19 will only use COVID-19 vaccines that receive full licensure from the Food and Drug Administration (FDA), in accordance with FDA-approved labeling and guidance.” The problem with this is that the Comirnaty vaccine product that was approved by the FDA is not available anywhere in the Military Health System. It is not even in production, according to the military’s TRICARE healthcare providers. If a soldier goes to a military hospital or a private provider to receive an approved Pfizer COVID vaccine, he will be administered the unapproved Pfizer-BioNTech vaccine which is a vaccine that is not approved but has been administered under an Emergency Use Authorization (EUA). We are told that this is but a brand name difference, that the formulation is the same, and they can be used interchangeably. But as the FDA was approving the Comirnaty product, they were renewing the authorization for the Pfizer-BioNTech product. If it’s just a matter of brand name, why issue an approval for one brand name and an EUA renewal for the other? This is because they are not actually the same.
Read More