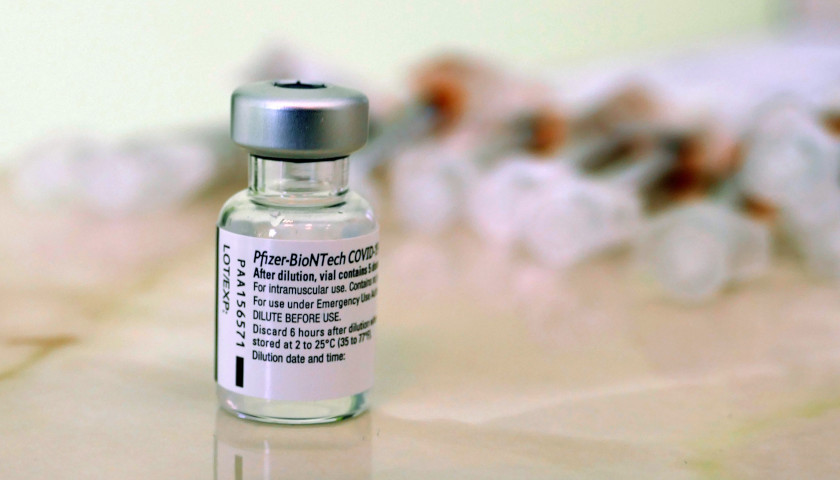
The Pfizer BioNTech COVID-19 vaccine authorized under the Emergency Use Authorization (EUA) has a different label than the FDA-approved Comirnaty label for the vaccine, and Pfizer has said they will continue to distribute the vaccine made under the earlier label until stocks run out.
The EUA was granted before the risk of myocarditis for men under 40, caused by the vaccine, was known, and the Comirnaty package insert found on the FDA website includes warnings about the rare side effect. A fact sheet distributed with the EUA vaccine also includes a warning about the risk.
On Tuesday, an FDA official told The Virginia Star on background that the FDA has to ensure that EUA vaccine recipients are informed of the EUA, the extent and benefits of the vaccine, that the vaccine is optional, and of alternatives to the vaccine. Normally that data is communicated through a fact sheet for EUA vaccines. A package insert is used with fully approved vaccines like Comirnaty.
Read More