COVID-19 vaccine supporters are fond of sneering at public figures who have called for the Food and Drug Administration to pull or at least re-evaluate the safety of the increasingly unpopular therapeutics, such as Health and Human Services secretary nominee Robert F. Kennedy Jr., cardiologist Peter McCullough and Florida Surgeon General Joseph Ladapo.
Read MoreTag: Food and Drug Administration
Healthshare Org Urges Incoming Trump Admin to Make ‘Radical’ Change to FDA, Healthcare System
Solidarity HealthShare is calling on the incoming Trump Administration to fix the “nation’s broken healthcare system” with widespread changes to the Food and Drug Administration (FDA).
Read MoreLawmakers Demand Investigation into U.S. Drug Companies Who Reportedly Worked with Chinese Military
A group of bipartisan lawmakers are demanding an investigation into U.S. drug companies with reportedly troubling ties to China, according to a letter obtained by the Daily Caller News Foundation.
The lawmakers raised alarm to the Food and Drug Administration (FDA) in a Monday letter that they had identified some U.S. pharmaceutical companies as having worked with the Chinese military, raising concerns that U.S. intellectual property is being siphoned off by Beijing. The lawmakers also pointed out that several U.S. pharmaceutical companies also conducted clinical trials in the Xinjiang province of China, a region known for “genocide” against religious minorities, according to a letter sent by the lawmakers to the agency.
Read MoreSCOTUS Refused to Ban Federal Censorship Pressure; It Could Make Churches Complicit in Abortion
When the Supreme Court reversed a preliminary injunction against several federal agencies and officials in June for “coerc[ing] or significantly encourag[ing]” tech platforms to suppress content, Washington state saw a new way to protect its mandatory abortion coverage in maternity healthcare plans from religious freedom challenges.
Five years into a lawsuit by Cedar Park Assembly of God against SB 6219, which includes criminal penalties up to prison, the Evergreen State argues that insurers won’t necessarily offer abortion-free plans if the court permanently bars it from enforcing surgical- and chemical-abortion coverage against such religious ministries that are opposed to abortion.
Read MoreFDA Knew ‘Gender Affirming’ Puberty Blockers Increase ‘Suicidality’ in 2017, Promotes Them Today
Five months before the Food and Drug Administration issued a health warning on puberty blockers widely used off-label to treat minors with gender confusion, undermining a Department of Health and Human Services office that claimed “early gender affirming care is crucial to overall health and well-being,” an FDA leader acknowledged other health concerns.
Pediatric patients exposed to “gonadotropin-releasing hormone agonists,” most with central precocious puberty (CPP) and “a handful … transgender kids using the drugs off-label,” had an “increased risk of depression and suicidality, as well as increased seizure risk,” Division of General Endocrinology clinical team leader Shannon Sullivan told colleagues.
Read MoreLouisiana Abortion Pill Reclassification Bill Heads to Governor’s Desk
The Louisiana state Senate approved a bill on Thursday that would place two abortion pills on the state’s list of controlled dangerous substances, sending the legislation to the governor’s desk for his signature.
The state’s House of Representatives passed the bill on Tuesday, which could make possession of the drugs a crime punishable by jail time or a fine. Surgical and medical abortions are already illegal in the southern state except in extreme cases, meaning it is already difficult to obtain the drugs legally. But now the possession itself without a prescription could get an individual up to five years in prison.
Read MoreFDA Threatens Endangered Species with Shoddy Abortion-Drug Reviews: Lance Armstrong Investigator
Federal public health officials created strange bedfellows among animal-welfare advocates, scientists and vaccine skeptics for allegedly cutting corners in viral and COVID-19 vaccine research and oversight, possibly engineering a pathogen, then a cure that’s worse for some.
The Food and Drug Administration may be creating another odd couple in a case at the Supreme Court: environmental and pro-life activists.
Read MoreFeds Conceal Details About Anti-Ivermectin Campaign in Response to Doctors’ Reinstated Lawsuit
The Food and Drug Administration wants to continue its selective promotion of off-label drug use: good for COVID-19 vaccines, bad for alternatives to those vaccines. It just doesn’t want the public to see its full reasoning for the latter.
The FDA and the Department of Health and Human Services filed a renewed motion to dismiss a lawsuit by doctors claiming the agencies have a practice of demonizing ivermectin by conflating its human and animal doses and using “command” language, such as “stop it,” to discourage using the anti-parasite drug against COVID.
Read MoreFDA Inspections of Foreign Drug Facilities Plummeted Since Before COVID-19, Study Shows
The Food and Drug Administration (FDA) has inspected fewer pharmaceutical manufacturing facilities compared to before the COVID-19 pandemic, with foreign facilities, including those from China, seeing the largest decreases, according to a study released in December.
In 2022, the total number of inspections of drug manufacturing establishments by the FDA decreased by 79% for foreign and 35% for domestic facilities compared to 2019, before the COVID-19 pandemic, according to a study by Emily Cuddy, Yun Peng Lu and David B. Ridley using data acquired through Freedom of Information Act requests. Despite the drop in inspections, there was no corresponding decrease in imports or manufacturing, while resources allocated by the FDA toward inspections surged per inspection.
Read MoreMore Restaurants, Bars Stock Up on Fentanyl, Opioid Overdose Reversal Drug as Deaths Soar
An increasing number of restaurants and bars across the country are keeping a stock of Naloxone, an antidote to fentanyl and opioid overdoses, according to The New York Times.
Local officials and nonprofit organizations are ramping up efforts to more bars and restaurants as overdoses become all too common in public spaces, according to the NYT. Between February 2022 and February 2023, there were more than 105,000 reported drug overdoses in the U.S., according to provisional data from the Centers for Disease Control and Prevention (CDC).
Read MoreAppeals Court Says FDA Denunciations of Ivermectin Look Like ‘Command,’ not Advice
The Food and Drug Administration (FDA) is claiming in federal court that it never told doctors not to prescribe ivermectin to treat COVID-19. Federal judges aren’t buying it, and state medical boards that rely heavily on FDA guidance continue to investigate doctors for such prescriptions.
Echoing a federal district judge nine months ago, a three-judge panel of the 5th U.S. Circuit Court of Appeals pressed a Justice Department lawyer to reconcile the FDA’s repeated public denunciations of ivermectin as an off-label COVID treatment with its insistence that the agency is not liable for resulting investigations of doctors who prescribe or promote it.
Read MoreWorld Health Organization Labels Aspartame as a Possible Cancer Cause, FDA Disagrees
A World Health Organization (WHO) committee has released a report that finds the well known sweetener aspartame is a possible cause of cancer.
The new classification is based on a review of “limited evidence.” The U.S. Food and Drug Administration (FDA), however, disagrees with the report released Thursday, according to NPR.
Read MoreCommentary: The FDA Must Partner with State AGs to Crack Down on Illegal Vapes and Keep Kids Safe
Millions of kids and teens in America are falling victim to an insidious campaign to get them hooked on illegal, disposable vapes that are made in China and intentionally marketed in youth-enticing flavors.
Read MoreSupreme Court Maintains Broad Access to Abortion Pill, Pending Litigation
The Supreme Court on Friday opted to preserve access to mifepristone while a challenge to the Food and Drug Administration’s approval of the drug makes its way through the courts. The Biden administration and mifepristone manufacturer Danco Laboratories had appealed to the court for relief. The court did not decide on the merits of the case, which will continue through the court system, the Associated Press reported.
Read MoreCommentary: After Decades of Outsourcing to China, the U.S. is Running Out of Children’s Antibiotics
Acute shortages of orally delivered amoxicillin, penicillin and other children’s antibiotics throughout the 2022 and 2023 cold and flu season have made it difficult for doctors to treat normal childhood illnesses like ear infections, bronchitis, strep throat and rarer cases of infections caused after suffering Respiratory Syncytial Virus (RSV), and also sickle cell disease—for months.
The Food and Drug Administration (FDA) issued a warning about the amoxicillin shortage in Oct. 2022 just at the start of the cold and flu season. But since then, no statement has been issued by President Joe Biden about what appears to be an underreported public health crisis.
Read MoreTop Epidemiologist Wants Pandemic Emergency Powers Ended, Insurer Death Data Released
One of the nation’s leading epidemiologists is declaring there is no basis for President Joe Biden to extend his emergency pandemic powers and that it is essential for insurers to release data showing deaths and injuries to those who have received COVID-19 vaccines.
Dr. Harvey Risch, professor emeritus at the Yale University School of Public Health, told Just the News on Friday evening that federal agencies have epically mishandled the pandemic strategy by substituting theories and politics for science.
Read MoreGrowing Body of Evidence Disputes Claims That Puberty Blockers Are Safe, Reversible
Puberty blockers are widely touted by doctors and transgender activists as a safe and fully reversible way to pause puberty for children with gender identity issues, but a growing body of evidence is challenging those claims, according to The New York Times.
The drug prevents the surge in bone density that would normally occur during puberty, and patients can see lifelong bone issues that are never resolved, according to the Monday NYT article. Medical professionals are also challenging claims that the drug is reversible, arguing instead that blocking puberty permanently cements a child’s transgender identity and puts them on a path to lifelong biomedical intervention.
Read MoreMiyares Joins Amicus Brief Supporting Decision to Vacate Travel Mask Mandate
Attorney General Jason Miyares joined an amicus brief opposing the Biden administration’s ongoing lawsuit over the CDC’s mask mandate for interstate travel. A district court vacated the requirement, but the CDC appealed, and the Health Freedom Defense Fund v. Biden case is now in the U.S. Court of Appeals for the 11th Circuit.
“Mask Mandates across the country have been lifted in virtually every aspect of daily life. For months, Americans have been traveling safely while making their own, autonomous decisions. The CDC mask mandate on public transportation, like air travel, is obsolete and no longer necessary – not to mention a clear example of federal overreach,” Miyares said in a press release.
Read MoreCommentary: The Feds Pile Up Vaccine ‘Adverse Event’ Reports as They Decry Scaremongering Elsewhere
Since the Food and Drug Administration authorized the first vaccines for COVID-19 in late 2020, the government and much of the media have insisted that the medicines developed in record time are safe and effective. Those who raised questions about them have been routinely dismissed as conspiracy theorists.
Read MoreFDA Vaccine Panel Recommends COVID Shots for Babies and Toddlers
The Food and Drug Administration’s (FDA) vaccine advisory panel unanimously voted Wednesday to recommend the Moderna and Pfizer vaccines for infants and young children despite an abundance of calls from physicians, children’s health organizations, and members of Congress to refrain from approving the shots for a population that shows the least risk for serious disease from COVID.
The Vaccines and Related Biological Products Advisory Committee (VRBPAC) voted, 21-0, in favor of recommending Emergency Use Authorization (EUA) for the Moderna and Pfizer mRNA COVID vaccines for infants, toddlers, and preschool-age children.
Read MoreDoctor Crashes FDA Meeting and Shares the Whistleblower Story They Ignored
A doctor “crashed” a Food and Drug Administration’s meeting with outside vaccine experts earlier this week, to share a whistleblower’s story about the data integrity issues that plagued one of Pfizer’s clinical trials.
In September of 2020, a researcher from an organization testing Pfizer’s vaccine at several sites in Texas, emailed a complaint to the FDA, informing the agency of the company’s dangerously shoddy research practices. The FDA took no action on her email, and Pfizer continues to use the company.
Read MorePfizer CEO Calls for Another Booster Shot for All Americans
On Sunday, the chief executive officer of Pfizer said that Americans should be prepared to receive a second booster shot of the Coronavirus vaccine, which would mark the fourth overall shot that has been forced on the American public.
As reported by Politico, Albert Bourla made his remarks in an interview with CBS’ Margaret Brennan, where he said that his company was preparing to submit “a significant package of data about the need for a fourth dose” to the Food and Drug Administration (FDA).
Read MoreAbortion Pills Now More Common Than Surgical Abortions
Medication-induced abortions accounted for 54% of all abortions in the U.S. in 2020, according to the Guttmacher Institute.
Abortion pills have grown in popularity since they were first introduced in 2000, the Guttmacher Institute reported. And rules requiring women to receive their first two abortion pills at a clinic or doctor’s office were lifted during the pandemic, allowing women to speak with doctors via “telemedicine” and get the pills by mail, The New York Times reported.
Read MoreFDA Executive Says on Hidden Camera That Yearly COVID Shots Will Be Mandatory for All Americans – Including Toddlers
The Biden administration plans to make yearly COVID shots mandatory for all Americans, including young children, a Food and Drug Administration executive told a Project Veritas undercover journalist on hidden camera.
In the sting video released on Tuesday, Christopher Cole, Executive Officer of Countermeasures Initiative for the FDA, said: “Biden wants to inoculate as many people as possible.” According to Project Veritas president James O’Keefe, Cole has “over 20 years experience” at the FDA, and “claims to be directly involved in the approval process.”
Read MoreFDA Announces Postponement of Approval of COVID Vaccine for Babies and Young Children
Pfizer and the Food and Drug Administration (FDA) said Friday they are delaying their plan for Pfizer’s Emergency Use Authorization (EUA) for its coronavirus vaccine for children under five years old due to insufficient data on the efficacy of a third dose.
Pfizer announced February 1 FDA had asked the drug company, and its partner BioNTech, to submit data on a COVID vaccine series for babies as young as six months old and young children up until age five.
Read MorePfizer Plan for COVID Vaccine Series for Babies of 6 Months Draws Fierce Controversy
Pfizer announced last week the Food and Drug Administration (FDA) had asked the drug company, and its partner BioNTech, to submit data on a COVID vaccine series for babies as young as 6 months old.
Albert Bourla, chairman and CEO of Pfizer, said in the statement:
As hospitalizations of children under 5 due to COVID-19 have soared, our mutual goal with the FDA is to prepare for future variant surges and provide parents with an option to help protect their children from this virus. Ultimately, we believe that three doses of the vaccine will be needed for children 6 months through 4 years of age to achieve high levels of protection against current and potential future variants. If two doses are authorized, parents will have the opportunity to begin a COVID-19 vaccination series for their children while awaiting potential authorization of a third dose.
Read MoreGovernor Ron DeSantis Shreds Biden over Decision to Revoke Emergency Use Authorization for Monoclonal Antibody Treatments
Florida Governor Ron DeSantis shredded President Joe Biden’s administration over the decision to revoke the emergency use authorization for Regeneron and Eli Lilly monoclonal antibody treatments.
According to the Food and Drug Administration, the treatments are not effective against the Omicron variant. Because the variant accounts for most cases of the coronavirus across the country, leaders of the agency limited its use.
Read MoreCommentary: What Reverend Martin Luther King, Jr. Would Say About Biden’s New COVID-19 Policy
Given the Biden administration’s recent effort to prioritize COVID-19 treatments based on race, it is more important than ever that we remember – and practice – the teachings of Reverend Martin Luther King Jr.
Last week, the Food and Drug Administration released new guidance to medical professionals which listed “race or ethnicity” as high risk factors for doctors to consider when prescribing a new monoclonal antibody known as Sotrovimab. Other high-risk factors included obesity, pregnancy, and other health conditions which would make a person less able to fight the virus. The new guidance means a person’s race could qualify him or her for treatment ahead of others who need the drugs.
Biden administration officials have cited high rates of diabetes and other health issues which are prevalent in non-white and non-Hispanic communities as reasons to include the new criteria. Officials in New York and Minnesota have also prioritized treating non-white patients, but they have more overtly cited historic health care disparities as a justification.
Read MoreFDA Claims It Needs 55 Years Before Revealing Data on Approval of Pfizer Vaccine
On Monday, the Food and Drug Administration (FDA) requested that the courts allow the agency to wait until the year 2076 to release all of the relevant documents regarding the approval of the vaccine developed by Pfizer and BioNTech, as reported by the Daily Caller.
The FDA made its request after a lawsuit was filed against the agency by the group Public Health Medical Professionals for Transparency (PHMPT). The PHMPT had previously made a Freedom of Information Act (FOIA) request on September 9th asking for the release of the vaccine approval documents; after the FDA denied the request, the group filed its lawsuit on September 16th.
The FDA concluded that there were roughly 329,000 pages in total that would qualify under this FOIA request. In its appeal to the courts, the agency said that, at most, employees would be able to “process and produce the non-exempt portions of responsive records at a rate of 500 pages per month.” Under this process, the FDA said that it would hand over prioritized documents to the plaintiff, and release non-exempt documents on a “rolling basis.”
Read MoreBiden Announces Former Obama FDA Commissioner Robert Califf to Lead FDA Again
President Joe Biden on Friday announced the nomination of Robert Califf to head up the Food and Drug Administration again, urging the Senate to quickly confirm him a second time.
Califf, who served in the same role near the end of then-President Barack Obama’s second term, is “one of the most experienced clinical trialists in the country, and has the experience and expertise to lead the Food and Drug Administration during a critical time in our nation’s fight to put an end to the coronavirus pandemic,” Biden said in a statement announcing the pick.
“I am confident Dr. Califf will ensure that the FDA continues its science and data driven decision-making,” the president added, pointing out that Califf enjoyed “strong bipartisan support in the Senate in 2016.”
Read MoreCommentary: Defense Department Pulls a Bait and Switch on Vaccines
On August 24, Secretary of Defense Lloyd Austin issued a memo to senior Pentagon leadership announcing that he was implementing a mandatory COVID-19 vaccination policy for all military service members. The day before, the FDA had issued full authorization to Pfizer for their Comirnaty COVID-19 vaccine product (the nomenclature of which is meant to be a mashup of the words “COVID”, “mRNA”, and “community”) . At first glance it would seem that the mandatory vaccination policy, while scientifically unsound and strategically foolish, was at least a policy being implemented according to both the letter of the directive and in accordance with the law. But a further examination of the facts and the manner in which this order is being implemented makes clear that the military’s implementation of this order is illegal and highly unethical.
In the memo, Secretary Austin issued a directive and a promise, that “Mandatory vaccination against COVID-19 will only use COVID-19 vaccines that receive full licensure from the Food and Drug Administration (FDA), in accordance with FDA-approved labeling and guidance.” The problem with this is that the Comirnaty vaccine product that was approved by the FDA is not available anywhere in the Military Health System. It is not even in production, according to the military’s TRICARE healthcare providers. If a soldier goes to a military hospital or a private provider to receive an approved Pfizer COVID vaccine, he will be administered the unapproved Pfizer-BioNTech vaccine which is a vaccine that is not approved but has been administered under an Emergency Use Authorization (EUA). We are told that this is but a brand name difference, that the formulation is the same, and they can be used interchangeably. But as the FDA was approving the Comirnaty product, they were renewing the authorization for the Pfizer-BioNTech product. If it’s just a matter of brand name, why issue an approval for one brand name and an EUA renewal for the other? This is because they are not actually the same.
Read MoreSidney Powell Sues Defense Department over Vaccine Mandate
Former Trump attorney Sidney Powell announced Wednesday that she is suing the Defense Department in regards to their vaccine mandate.
According to The Hill, Powell is representing the Texas-based group “Defending the Republic” in a lawsuit against Defense Secretary Lloyd Austin in regards to the military’s mandatory vaccination requirements.
Read MoreTelehealth Abortions Are Available to Virginians
Planned Parenthood of Metropolitan Washington, D.C., (PPMW) is now providing telehealth abortions to people with addresses in Virginia, Maryland, and D.C., according to a September 10 press release. After a phone screening and an online consultation, PPMW mails abortion drugs to the patient. Total cost for the service is $525, including a follow-up consultation and pregnancy test.
Read MoreLaw Professor Accuses University of Violating Federal Trade Commission Rules with Mask Mandate
A business law professor who has been put on paid leave for refusing to wear a mask in class is defending his actions with an unexpected authority: the Federal Trade Commission (FTC).
“[B]y requiring employees to wear a mask, you are promoting the idea that the mask can prevent or treat a disease, which is an illegal deceptive practice,” David Clements, who teaches consumer law at New Mexico State University (NMSU), told provost Carol Parker in a Sept. 13 letter.
Read MoreFDA Says It Does Not Buy Fetal Tissue – Any More
The Food and Drug Administration assured the Daily Caller News Foundation Friday that it has not entered into any contracts “for the purchase of human fetal tissue” since 2018.
The agency’s response follows the release of documentation obtained by Judicial Watch showing that the FDA procured fetal organs, tissue, and heads for research that involved “humanized mice.” Previous documents uncovered by Judicial Watch found that the FDA requested “fresh and never frozen” fetal organs.
“I’ve been doing this for 23 years. These documents we’ve gotten from the FDA and our other lawsuit…they are the worst things I’ve ever seen,” Judicial Watch President Tom Fitton told the Daily Caller News Foundation Friday. “The most troubling documents I’ve ever seen.”
Read MoreFDA Will Need More Time to Decide If Juul Can Sell E-Cigarettes
Health officials delayed a decision Thursday on whether e-cigarettes made by Juul and other top companies can stay on the U.S. market.
The Food and Drug Administration (FDA) said it needs longer than the Thursday deadline to determine if Juul and other select companies’ products can continue to be sold in the U.S., according to a press release.
Read MoreReport: Top Health Officials Tell White House to Pause Vaccine Booster Plan
Top U.S. health officials told the White House pandemic coordinator on Thursday to scale back the Biden administration’s plan to administer the coronavirus booster shots to individuals in September, The New York Times reported.
Dr. Janet Woodcock, the acting commissioner of the Food and Drug Administration (FDA), and Dr. Rochelle P. Walensky, the director of the Centers for Disease Control and Prevention (CDC), told White House Coronavirus Response Coordinator Jeffrey D. Zients that they need more time to collect and analyze the necessary data relating to the booster shots, The New York Times reported.
The doctors told Zients that their agencies might be able to determine whether to recommend boosters for recipients of the Pfizer-BioNTech vaccine in the coming weeks, according to the Times.
The two doctors presented their argument to Zients at a meeting on Thursday. It is unclear how Zients responded to the news.
Read MoreFDA Bans 55,000 Flavored E-Cigarette Products
The U.S. Food and Drug Administration banned 55,000 e-cigarette products on Thursday for their failure to prove they didn’t pose a threat to public health.
The FDA announced that thousands of products from three vape companies, JD Nova Group LLC, Great American Vapes, and Vapor Salon, didn’t prove the benefit to adult smokers negated the “well-documented, alarming levels of youth use of such products.”
Read MoreTexas Gov. Abbott Bans Vaccine Mandates Statewide Despite FDA Approval
Texas Gov. Greg Abbott on Wednesday banned government-issued vaccine mandates despite the Food and Drug Administration’s recent approval of Pfizer’s coronavirus vaccine.
Abbott’s executive order applies to all government-run entities with the exception of nursing homes and assisted living facilities. “Vaccine requirements and exemptions have historically been determined by the legislature, and their involvement is particularly important to avoid a patchwork of vaccine mandates across Texas,” Abbott said in an accompanying statement.
Read MoreFully Vaccinated Do Not Need Booster for Delta Variant, CDC and FDA Say
Fully vaccinated Americans do not need to receive a booster shot to protect against the Delta variant, the Centers for Disease Control and Prevention and Food and Drug Administration said in a press release.
“People who are fully vaccinated are protected from severe disease and death, including from the variants currently circulating in the country such as Delta,” a joint statement said on Thursday.
Read MoreStudy Finds Novavax COVID-19 Vaccine 90 Percent Effective
Novavax announced on Monday that its two-dose COVID-19 vaccine is 90% effective, according to a press release on Novavax’s website.
The phase-3 trial enrolled 29,960 participants ages 18 and older in the U.S. and Mexico. The study found that 77 of the participants tested positive for COVID-19, with 63 testing positive in the placebo group and 14 in the vaccine group, according to the press release.
“Today, Novavax is one step closer to addressing the critical and persistent global public health need for additional COVID-19 vaccines. These clinical results reinforce that NVX-CoV2373 is extremely effective and offers complete protection against both moderate and severe COVID-19 infection,” Stanley C. Erck, President, and CEO of Novavax said in the press release.
Read MoreAt Least 40 Percent of NIAID and FDA Employees Have Not Been Vaccinated, According to Fauci and Marks
At least 40 percent of National Institute of Allergy and Infectious Diseases (NIAID), and Food and Drug Administration (FDA) employees are refusing to get the COVID-19 vaccine according to NIAID Director Dr. Anthony Fauci, and FDA official Dr. Peter Marks.
During a Senate Health, Education, Labor, and Pensions Committee hearing Tuesday on efforts to combat the COVID-19 pandemic, Senator Richard Burr (R-Va.) asked Fauci, Marks, and Centers for Disease Control and Prevention (CDC) Director Dr. Rochelle Walensky what percentage of their own employees were vaccinated.
Both Fauci and Marks estimated that a little more than half—perhaps around 60 percent of their employees—have been vaccinated. Walensky waffled, saying only that she was “encouraging employees to get vaccinated,” but couldn’t say how many have actually done so.
Read MoreVirginians 16 and Older Eligible for COVID-19 Vaccine
All Virginians 16 years old and older are now eligible to receive the COVID-19 vaccine, as of Sunday.
“Over the past few months, we have made tremendous progress vaccinating Virginians as quickly, safely, and equitably as possible, and we need to keep up the good work,” Governor Ralph Northam said in a press release.
Read MoreCoronavirus Vaccines Can Guard Against New, More Contagious UK Strain, Experts Say
The two coronavirus vaccines that have been approved for emergency use authorization in the U.S. will be able to combat a new, more contagious strain of the virus in the U.K., experts said Monday.
Vaccines made by pharmaceutical companies Pfizer and Moderna will be effective against the new strain, which is “very similar” to previous strains at the genetic level, University of Washington’s Institute for Health Metrics and Evaluation affiliate assistant professor Vin Gupta told CNBC. The Food and Drug Administration has approved both vaccines for emergency use authorization after large-scale human trials showed efficacy of more than 90%.
Read MoreJudge Theodore Chuang Rules Women Can Get Abortion Pill Without Doctor Visit
A federal judge agreed Monday to suspend a rule that requires women during the COVID-19 pandemic to visit a hospital, clinic or medical office to obtain an abortion pill.
U.S. District Judge Theodore Chuang, an Obama appointee based in Maryland, concluded that the “in-person requirements” for patients seeking medication abortion care impose a “substantial obstacle” to abortion patients and are likely unconstitutional under the circumstances of the pandemic.
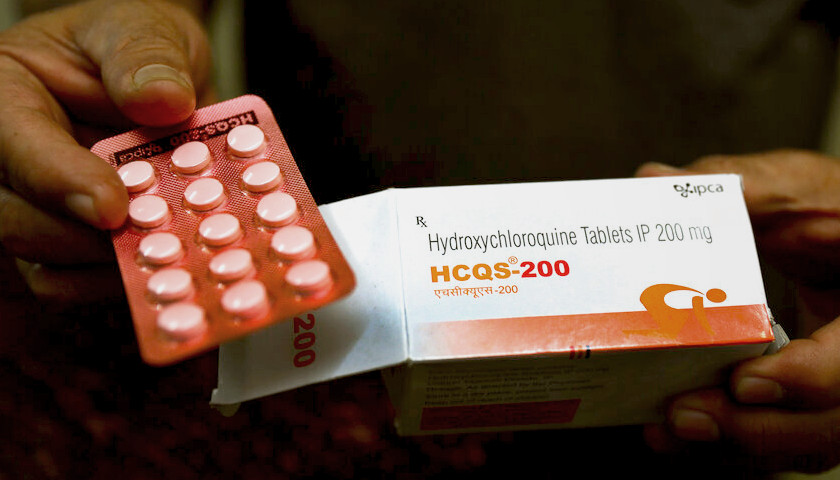
Read MoreFDA Revokes Emergency Use of Malaria Drugs to Treat Coronavirus, Cites ‘Potential Risks’
U.S. regulators on Monday revoked emergency authorization for malaria drugs promoted by President Donald Trump for treating COVID-19 amid growing evidence they don’t work and could cause deadly side effects.
The Food and Drug Administration said the drugs hydroxychloroquine and chloroquine are unlikely to be effective in treating the coronavirus. Citing reports of heart complications, the FDA said the drugs’ unproven benefits “do not outweigh the known and potential risks.”
Read More